Question 1: Convert the following temperature to the Celsius scale.
(a) 293 K (b) 470 K
Answer: (a) Temperature in Celsius scale = Temperature in Kelvin scale - 273
⇒ 293 K = 293 K – 273 = 20⁰C
(b) Temperature in Celsius scale = Temperature in Kelvin scale - 273
⇒ 470 K = 470 K – 273 = 197⁰C
Question 2: Convert the following temperature to the Kelvin scale.
(a) 25⁰C (b) 373⁰C
Answer: (a) Temperature in Kelvin scale = Temperature in Celsius scale + 273
= 25⁰C + 273 = 298 K
Answer: (b) Temperature in Kelvin scale = Temperature in Celsius scale + 273
= 373⁰C + 273 = 646 K
Question 3: Give reason for the following observations.
(a) Naphthalene balls disappear with time without leaving any solid.
Answer: Naphthalene ball is a sublimate and a sublimate turns into vapour without changing into liquid. Thus, naphthalene balls disappear with time without leaving any solid.
(b) We can get the smell of perfume sitting several meters away.
Answer: Perfume turns into gas at room temperature. The vapour of perfume travels up to several meters because of diffusion. That’s why we can get the smell of perfume sitting several meters away.
Question 4: Arrange the following substances in increasing order of forces of attraction between the particles – Water, Sugar, Oxygen.
Answer: Oxygen < Water < Sugar
Explanation: Oxygen is a gas, thus force of attraction is negligible between particles. Water is a liquid, thus force of attraction between particles is more than liquid and less than solid. Sugar is a solid, thus force of attraction between particles is greatest.
Question 5: What is the physical state of water at (a) 25⁰C (b) 0⁰C (c) 100⁰C
Answer: (a) At 25⁰C – water is in liquid state.
(b) At 0⁰C – water is in solid state.
At 100⁰C – water is in transition state, i.e. in liquid and gas both.
Question 6: Give two reasons to justify;
(a) Water at room temperature is a liquid.
Answer: At room temperature:
- Water has definite volume, but not definite shape as it takes the shape of the container in which it is kept.
- Water flows at room temperature.
(b) An iron almirah is a solid at room temperature.
Answer: An iron almirah is a solid at room temperature because:
- It has definite shape.
- It has definite volume.
Question 7: Why is ice at 273K more effective in cooling than water at the same temperature.
Answer: At 273K ice requires more latent heat to melt into water, while water at 273K requires less latent heat; to come to the room temperature. So, ice at 273 K is more effective in cooling than water at the same temperature.
Question 8: What produces more severe burns, boiling water or steam?
Answer: Steam produces more severe burns than boiling water, because steam has more latent heat than boiling water.
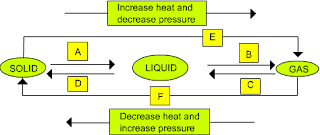
Question 9: Name A,B,C,D,E and F in the following diagram showing change in its state.
Answer:
A – Heating - Melting
B – Heating - Vapourisation
C – Cooling – Condensation - Liquefaction
D – Cooling – Freezing
E – Sublimation
F – Solidification
No comments:
Post a Comment